Research
Our group’s interest is in nuclear mechanisms that regulate changes of cellular identity during stem cell differentiation and specify the diverse cell types of the body (Figure 1). We have used the mammalian dosage compensation process, X inactivation, as an experimentally tractable system for studying the developmentally regulated establishment of silent chromatin. This has put us in a position to apply previously generated tools for studying aspects in stem cell biology. Using state of the art genetic tools we hope to uncover fundamental pathways of cell differentiation.
Figure 1 - Cell identity in development
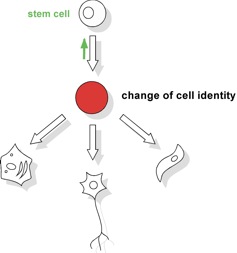
In mammals over 200 different types of cells form the tissues of the body. These cells must stably maintain their identity to continuously support organ function and prevent disease. Ultimately, however, all cells have to be generated from a single cell through development, and many of these cells are constantly replenished in adulthood. This involves the differentiation of stem cells and immature cell types and goes along with a change in cell identity. Thus, at some point in differentiation cell identity becomes unstable such that a change from a stem cell to a functional differentiated cell can be accomplished. Little is known how epigenetic control of the genome is accomplished during a change in cell identity. Of note also adult stem cells are generated from embryonic progenitor cells (green arrow).
Understanding the establishment of epigenetic patterns
Using tools derived from the mammalian dosage compensation system can be useful for characterizing the establishment of epigenetic patterns. X inactivation is initiated in early embryonic cells and balances gene copy numbers between males and female by inactivation of one of the two X chromosomes in female cells. In mice, X inactivation is triggered by the 17 kb long noncoding Xist RNA that accumulates over the inactive X chromosome (Figure 2). During cell differentiation the Xi becomes further modified resulting in a stably silenced and largely heterochromatic chromosome. Notably, X inactivation highlights a powerful mechanism for gene regulation in cis as a female cell nucleus contains an active (Xa) and an Xi that can be of identical DNA sequence yet are in different transcriptional states. It is expected that exploring the mechanism behind X inactivation will lead to discovery of gene regulation pathways that also function more broadly in mammalian development.
Figure 2 - Xist RNA associates with a chromosome in cis
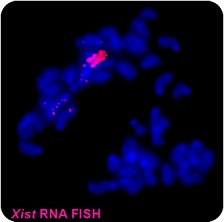
RNA FISH experiment showing Xist RNA (red) associated within a condensed chromosome (DNA in blue). Xist accumulates within the chromosomes territory from which it is transcribed and mediates gene repression. Xist serves as a paradigm for understanding the role of RNA in establishing epigenetic patterns, chromatin organization and regulating gene expression in the mammalian cell nucleus.
Characterization of epigenetic transitions in stem cell differentiation
The function of Xist for initiating gene repression depends on the cell type. Silencing is established in cells of the early embryo and in specific haematopoietic progenitors (Savarese et al., 2006). Yet, somatic tissues cells do not represent a silencing context for Xist. This suggests that pathways conferring gene silencing function to Xist are under developmental regulation. Analysis of the context for Xist mediated silencing in haematopoietic and tumor cells has led us to identify SATB1 as a protein that is linked to the potential of Xist to induce chromosome-wide gene silencing (Agrelo, et al., 2009). In extension to our analysis of the haematopoietic system we are performing an analysis of Xist function in other stem cell niches to investigate how general a switch in epigenetic context is for adult stem cell differentiation.
In a separate line of investigation, we examine the reactivation of the Xi in development and adult stem cell differentiation (Ohhata et al, 2011). The inactive X chromosome in female cells is a hallmark of all female somatic cells. Genes on the inactive X chromosome are stably silenced by a number of epigenetic mechanisms including DNA methylation and histone deacetylation. Reactivation of the inactive X chromosome is observed when induced pluripotent stem cells are generated. Understanding the chromatin configuration of the Xi will provide insight how the stability of epigenetic silencing is regulated in a development.
The function of Polycomb complexes in embryonic stem cell differentiation
The Polycomb group (PcG) of genes are important epigenetic regulators during development. Polycomb complexes establish chromatin modifications for maintaining gene repression and are essential for development in flies and mammals. In mice, Polycomb group (PcG) proteins are chromatin regulators with function in Hox gene regulation, development and tumorigenesis, and contribute to genomic imprinting and dosage compensation. Two distinct PcG complexes with histone modifying activities have been identified. Polycomb repressive complex 2 (PRC2) contains the PcG proteins Ezh2, Eed and Suz12 and mediates di- and tri-methylation of histone H3 lysine 27 (H3K27me3). PRC1 possesses histone H2A specific ubiquitination activity and contains the E3 ubiquitin ligase and PcG protein Ring1B. In mice, PcG complexes have crucial functions in development and it has been shown that mutations in Ezh2, Eed or Ring1B result in an early embryonic lethality after implantation.
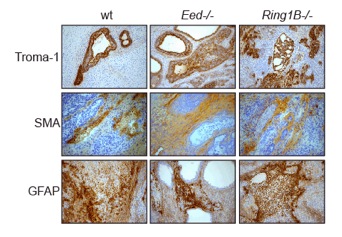
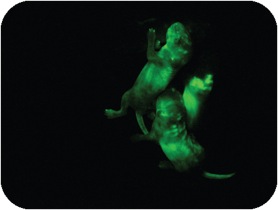
To understand the role of PcG complexes in cell differentiation, we have used pluripotent embryonic stem (ES) cells (Figure 3). Our data show that lineage specific genes are derepressed in ES cells lacking either the Ring1B or the Eed (Schoeftner et al. 2006; Leeb and Wutz 2007; Leeb et al. 2010). These transcriptional changes destabilize ES cells but are compatible with ES cell self renewal. Furthermore, ES cells lacking either PRC1 or PRC2 function are capable to differentiate in culture and form teratomas consisting of cells from the three germ layers and contribute to chimeric embryos (Figure 4) indicating that neither PRC1 nor PRC2 are absolutely required for cell differentiation.
We have further studied the combined mutation of Eed and Ring1B in mouse ES cells. Double deficient cells can be maintained in culture and can self renew (Leeb et al. 2010). However, differentiated cells are not maintained in culture in the absence of PRC1 and PRC2. On the molecular level the differentiation defect is caused by the derepression of a set of genes that is redundantly repressed by PRC1 and PRC2 in ES cells. Furthermore, we find that genomic repeats are Polycomb targets and show that in the absence of Polycomb complexes endogenous MLV elements can mobilize. These findings indicate a crucial role of the PcG system in cell differentiation in mammals and suggest a contribution of the PcG system to the defense against parasitic DNA. Genomic repeats could also potentially contribute to Polycomb mediated gene regulation.
Figure 3 - Histone modifications in Polycomb deficient ES cells
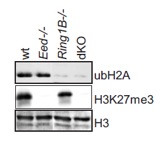
Western analysis of histone modifications in ES cell cultures of indicated genotypes shows the absence of Polycomb associated chromatin modifications.
Identification of epigenetic regulators through genetic screening in haploid ES cells
Genetic screens have facilitated the understanding of a wide range of pathways in genetically tractable model organisms such as fruit flies or the worm Caenorhabditis elegans. However, the diploid genomes of complex organisms have limited genetic approaches in biomedical model species such as in mice. To overcome this problem experimental induction of haploidy has been used in fish. In contrast, haploidy appears less compatible with development in mammals. We have developed haploid embryonic stem (ES) cells from mice and explored their application in forward genetic screening. Haploid ES cells can be established through the activation of unfertilized oocytes isolated from a variety of mouse strains including genetically modified mouse lines (Leeb and Wutz, 2011). In contrast to normal diploid cells haploid ES cells have a single set of chromosomes (Figure 5). Hence, the introduction of mutations into the genome of haploid cells has immediate phenotypic consequence and can be exposed to appropriately designed selection. In a small scale pilot screen the use of mouse haploid ES cells for the recovery of recessive autosomal mutations has been established. Importantly, haploid ES cells maintain a wide developmental potential in culture and can be used for the production of chimeric mice (Figure 6 and 7). Contribution to development is paralleled with a gain of a diploid karyotype. Notably, cells derived from haploid ES cells can enter the female germline and transmit genetic modifications to the next generation (Leeb et al., 2012). We aim to further explore haploid ES cells are a screening platform for for genetic dissection of gene regulation, signalling and metabolic pathways in mammalian development.
Figure 5 - Chromosome spread of haploid ES cells
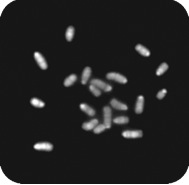
20 acrocentric chromosomes corresponding to the mouse haploid chromosome set can be distinguished suggesting a largely normal haploid karyotype.